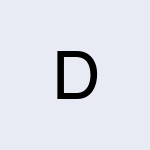Charge Technologist
Dynacare
Winnipeg, MB-
Number of positions available : 1
- Salary To be discussed
-
Permanent job
- Published on February 15th, 2025
-
Starting date : 1 position to fill as soon as possible
Description
POSITION TITLE:
Charge Technologist
INTERNAL CLOSING DATE:
Friday, February 21, 2025LOCATIONS:
King Edward100-830 King Edward Street
CLASSIFICATION:
Full-Time, Permanent
DEPARTMENT:
Microbiology
COMPENSATION:
As per Collective Agreement
WORKING SCHEDULE:
Monday to Friday, 8:00am to 16:00pm
(37.5 hours per week)
JOB SUMMARY
As a Charge Technologist, you will be responsible for ensuring the efficient operation and functionality of the operations in conjunction with the Manager, all in keeping with Dynacare’s mission and values.
MANDATE:
Performs a variety of routine laboratory procedures to obtain data for use in the diagnosis and treatment of disease.
KEY ACCOUNTABILITIES:
- Responsible for ensuring sample identification and preparation for testing are correct before commencing tests. Performs scheduled tests and measurements using a variety of manual and automated procedures.
- Sets up and adjusts laboratory equipment. Monitors supplies and identifies shortages and take timely and appropriate actions.
- Ensure that Quality Control checks are run, reviewed and appropriate actions taken when QC fails.
- Participates in all internal and external quality control programs.
- Ensures routine maintenance of laboratory equipment has been performed.
- Maintains confidentiality and accuracy of appropriate documents.
- Supervises the ordering of routinely used consumable supplies within the section.
- Supervises inventory of all supplies utilized in the section.
- Co-ordinates the “trouble-shooting”, maintenance, service and/or repair of laboratory equipment within the section as requested by staff.
- Is responsible for preparation and maintenance of procedure manuals in concert with Lab Ops Manager.
- Responsible for health and safety of employees by reporting hazards and ensuring compliance with established safe working practices
- Performs duties in accordance with established company processes and procedures.
- Ensure the efficient operation and functionality of the operations in conjunction with the Manager.
- Acts as first point of contact for operational and customer issues at designated locations.
- Assist the Manager in coordinating workflow, staff training, competencies and evaluation as instructed. Recommend changes to maintain high level of productivity in conjunction with the leadership of the Manager.
- Ensure all new and existing employees are properly trained including signing-off on competencies and other required documentation.
- Ensure that standards for technical efficiency, quality control and other government regulations are adhered to.
- Assist with the development, interpretation and implementation of related policies and procedures.
- Provide assistance to employees with the development, interpretation, implementation and updates of the department’s related policies, procedures, and manuals as required.
- Communicate all issues to Manager and when Manager is absent; all communications should be to the designated replacement. Report all resolved or unresolved issues and complaints to the Manager.
- Provide guidance and report to Manager any investigation, documentation and implementation of preventative measures for all workplace accidents, injuries, and hazards.
- Ensure completion of health and safety and 5S reports.
- Ensures that Quality Control/Quality Assurance is run according to department guidelines and with industry standards as well as signing on maintenance sheets.
- Assists Lab Ops Manager in determining appropriate staffing levels in the department.
- Other duties as required
SKILLED KNOWLEDGE REQUIREMENTS:
Knowledge
- Must be currently registered with CSMLS and CMLTM - Registered Medical Technologist, practicing designation in good standing.
- Experience working in Microbiology Department.
Technical Skills
- Knowledge of Quality Control documentation and procedures as well as CAP Program and requirements.
- Knowledge of Job Department processing.
- A minimum of five (5) years’ experience in a Clinical Laboratory is desired.
- Additional experience in a supervisory capacity may be an asset.
Social Processing Skills
- Ability to multitask and work in a team environment.
- Aptitude in critical thinking and decision making.
- Attention to detail and organizational skills are essential.
- Ability to function in a fast-paced environment.
- Excellent communication skills.
- Experience in word processing and Excel preferred.
- Ability to work independently within a group environment.
***Please refer to Article 2 and 7 of the Collective Agreement regarding temporary positions, job postings, promotions and transfers***
Requirements
undetermined
undetermined
undetermined
undetermined